When Does The Cytoplasm Divide

Steps of the jail cell cycle. The brake point occurs between the Mone and S phases of interphase. The Grand2-Chiliad checkpoint occurs between the Grandtwo and One thousand phases. The spindle checkpoint occurs during the Grand stage. Central cyclins associated with each phase are shown.
Cell cycle checkpoints are control mechanisms in the eukaryotic cell cycle which ensure its proper progression. Each checkpoint serves as a potential termination point forth the prison cell bike, during which the conditions of the cell are assessed, with progression through the diverse phases of the cell wheel occurring only when favorable weather are met. In that location are many checkpoints in the cell cycle,[i] but the three major ones are: the G1 checkpoint, also known as the Outset or brake checkpoint or Major Checkpoint; the G2/M checkpoint; and the metaphase-to-anaphase transition, also known as the spindle checkpoint.[2] Progression through these checkpoints is largely adamant by the activation of cyclin-dependent kinases by regulatory protein subunits called cyclins, different forms of which are produced at each stage of the cell bike to control the specific events that occur therein.[3] [4]
Background [edit]
All living organisms are the products of repeated rounds of cell growth and division.[5] During this process, known as the cell cycle, a prison cell duplicates its contents and then divides in two. The purpose of the cell cycle is to accurately duplicate each organism's DNA and and then divide the jail cell and its contents evenly betwixt the 2 resulting cells. In eukaryotes, the cell bicycle consists of 4 primary stages: Mi, during which a prison cell is metabolically agile and continuously grows; Due south phase, during which DNA replication takes identify; G2, during which cell growth continues and the prison cell synthesizes various proteins in preparation for segmentation; and the K (mitosis) phase, during which the duplicated chromosomes (known every bit the sister chromatids) carve up into ii daughter nuclei, and the cell divides into two daughter cells, each with a full re-create of Deoxyribonucleic acid.[6] Compared to the eukaryotic cell wheel, the prokaryotic prison cell cycle (known as binary fission) is relatively simple and quick: the chromosome replicates from the origin of replication, a new membrane is assembled, and the cell wall forms a septum which divides the jail cell into two.[7]
Every bit the eukaryotic cell cycle is a complex process, eukaryotes have evolved a network of regulatory proteins, known as the cell bicycle control system, which monitors and dictates the progression of the cell through the cell cycle.[five] This system acts like a timer, or a clock, which sets a stock-still amount of fourth dimension for the cell to spend in each phase of the cell cycle, while at the same time information technology too responds to data received from the processes information technology controls. The prison cell cycle checkpoints play an of import role in the control system past sensing defects that occur during essential processes such equally DNA replication or chromosome segregation, and inducing a cell cycle arrest in response until the defects are repaired.[8] The main mechanism of activeness of the jail cell bicycle checkpoints is through the regulation of the activities of a family of protein kinases known as the cyclin-dependent kinases (CDKs), which demark to different classes of regulator proteins known every bit cyclins, with specific cyclin-CDK complexes existence formed and activated at dissimilar phases of the jail cell cycle. Those complexes, in turn, activate different downstream targets to promote or prevent prison cell cycle progression.[nine]
G1 (restriction) checkpoint [edit]
The G1 checkpoint, likewise known as the restriction bespeak in mammalian cells and the start point in yeast, is the point at which the cell becomes committed to entering the cell cycle. As the cell progresses through G1, depending on internal and external weather, information technology can either delay G1, enter a quiescent state known as G0, or proceed by the restriction point.[5] Deoxyribonucleic acid harm is the principal indication for a jail cell to "restrict" and not enter the cell cycle. The decision to commit to a new circular of cell division occurs when the cell activates cyclin-CDK-dependent transcription which promotes entry into South stage. This bank check signal ensures the further process.[x]
During early G1, there are 3 transcriptional repressors, known equally pocket proteins, that bind to E2F transcription factors. The E2F gene family is a group of transcription factors that target many genes that are important for control of the cell bicycle, including cyclins, CDKs, checkpoint regulators, and DNA repair proteins. Misregulation of the E2F family is often plant in cancer cases, providing evidence that the E2F family is essential for the tight regulation of DNA replication and partitioning.[10] The 3 pocket proteins are Retinoblastoma (Rb), p107, and p130, which bind to the E2F transcription factors to foreclose progression past the G1 checkpoint.
The E2F cistron family contains some proteins with activator mechanisms and some proteins with repressing mechanisms. P107 and p130 human action as co-repressors for E2F 4 and E2F 5, which work to repress transcription of G1-to-S promoting factors. The third pocket protein, Rb, binds to and represses E2F ane, E2F 2, and E2F iii, which are the E2F proteins with activating abilities.[10]
Positive feedback plays an essential role in regulating the progression from G1 to Southward stage, particularly involving the phosphorylation of Rb by a Cyclin/CDK poly peptide circuitous. Rb without a phosphate, or unphosphorylated Rb, regulates G0 cell wheel exit and differentiation. During the beginning of the G1 phase, growth factors and DNA impairment signal for the ascension of cyclin D levels, which and then binds to Cdk4 and Cdk6 to class the CyclinD:Cdk4/6 complex.[eleven] This complex is known to inactivate Rb by phosphorylation. However, the details of Rb phosphorylation are quite complex and specific compared to previous knowledge about the G1checkpoint. CyclinD:Cdk4/6 places only one phosphate, or monophosphorylates, Rb at one of its 14 accessible and unique phosphorylation sites. Each of the fourteen specific mono-phosphorylated isoforms has a differential binding preference to E2F family members, which probable adds to the diversity of cellular processes within the mammalian body.[eleven]
E2F iv and E2F 5 are dependent on p107 and p130 to maintain their nuclear localization. However, Cyclin D:Cdk 4/6 also phosphorylates p107 and p130, a process which releases their demark from E2F iv and 5 (which then escape to the cytoplasm), and allowing for E2F i-3 to bind to the DNA and initiate transcription of Cyclin E.[10] Rb proteins maintain their mono-phosphorylated land during early G1 phase, while Cyclin Eastward is accumulating and binding to Cdk2.
CyclinE:Cdk2 plays an additional important phosphorylation part in the G1-to-S transition. Particularly, CyclinE:Cdk2 promotes a positive feedback loop which creates an "all or zilch" switch. In many genetic control networks, positive feedback ensures that cells do not slip back and along between prison cell wheel phases [12] Cyclin E:Cdk2 gain to phosphorylate Rb at all of its phosphorylation sites, also termed "hyper-phosphorylate", which ensures complete inactivation of Rb. The hyper phosphorylation of Rb is considered the belatedly G1 restriction point, after which the cell cannot go backwards in the prison cell wheel. At this point, E2F 1-three proteins bind to DNA and transcribe Cyclin A and Cdc 6.[11]
Cyclin-dependent kinase inhibitor 1B (CDKN1B), also known as p27, binds to and prevents the activation of CyclinE:Cdk2 past inhibition. Withal, equally Cyclin A accumulates and binds to Cdk2, they grade a complex and inhibit p27. The G1 phase cyclin-dependent kinase works together with Due south stage cyclin-dependent kinase targeting p27 for degradation. In plough, this allows for full activation of Cyclin A:Cdk2, a complex which phosphorylates E2F 1-three initiating their disassociation from the Dna promoter sites. This allows E2F 6-viii to bind to the DNA and inhibit transcription.[10] The negative feedback loop used to successfully inhibit the inhibitor, p27, is some other essential process used by cells to ensure mono-directional movement and no backtrack through the cell cycle.
When Deoxyribonucleic acid damage occurs, or when the jail cell detects any defects which necessitate it to delay or halt the cell bike in G1, abort occurs through several mechanisms. The rapid response involves phosphorylation events that initiate with either kinase ATM (Ataxia telangiectasia mutated) or ATR (Ataxia Telangiectasia and Rad3 related), which act every bit sensors, depending on the blazon of impairment. These kinases phosphorylate and activate the effector kinases Chk2 and Chk1, respectively, which in turn phosphorylate the phosphatase Cdc25A, thus mark it for ubiquitination and degradation. Every bit Cdc25A activates the previously mentioned cyclin E-CDK2 complex by removing inhibitory phosphates from CDK2, in the absence of Cdc25A, cyclin Eastward-CDK2 remains inactive, and the prison cell remains in G1.
To maintain the arrest, another response is initiated, by which Chk2 or Chk1 phosphorylate p53, a tumor suppressor, and this stabilizes p53 by preventing it from binding Mdm2, a ubiquitin ligase which inhibits p53 past targeting it for degradation. The stable p53 then acts a transcriptional activator of several target genes, including p21, an inhibitor of the G1-to-South promoting circuitous cyclin Eastward-CDK2. In addition, some other mechanism by which p21 is activated is through the accumulation of p16 in response to Deoxyribonucleic acid harm. p16 disrupts cyclin D-CDK4 complexes, thus causing the release of p21 from the complexes, which leads to the dephosphorylation and activation of Rb, which allows Rb to bind and inhibit E2F i-3, thus keeping the jail cell from transitioning to S phase.[13] Recently, some aspects of this model have been disputed.[14]
G2 checkpoint [edit]
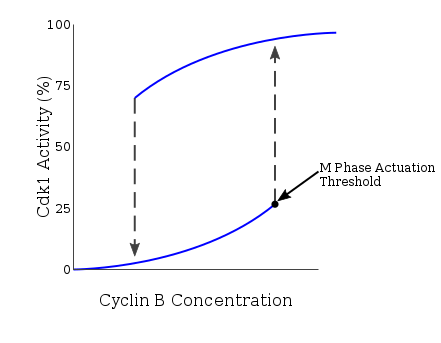
Mitotic Cyclin Concentration shows hysteresis and bistability relative to Cdk1 Activation
Post-obit Deoxyribonucleic acid replication in S phase, the prison cell undergoes a growth stage known as G2. During this time, necessary mitotic proteins are produced and the cell is once more subjected to regulatory mechanisms to ensure proper status for entry into the proliferative Mitotic (Chiliad) phase. Multiple mechanistic checkpoints are involved in this transition from G2 to Thou, with a common uniting cistron of cyclin-Cdk activeness.
Although variations in requisite cyclin-Cdk complexes be across organisms, the necessity of the kinase activity is conserved and typically focuses on a single pairing. In fission yeast three unlike forms of mitotic cyclin exist, and six in budding yeast, nevertheless the primary cyclin utilized is cyclin B.[15] Cyclin B will serve as reference for give-and-take of the G2/1000 checkpoint transition.
Similar to S Stage, G2 experiences a DNA harm checkpoint. The jail cell is once again examined for sites of Dna damage or incomplete replication, and the kinases ATR and ATM are recruited to impairment sites. Activation of Chk1 and Chk2 as well transpire, as well as p53 activation, to induce cell cycle arrest and halt progression into mitosis. An additional component of S phase, the Pre-Replicative Complex, must be inactivated via cyclin B-Cdk1 phosphorylation.[16]
As these previous checkpoints are assessed, G2 protein accumulation serves to actuate cyclinB-Cdk1 action via multiple mechanisms. CyclinA-Cdk2 activates Cdc25, an activator of cyclinB-Cdk1, which then deactivates the cyclinB-Cdk1 inhibitor, Wee1. This results in a positive feedback loop, significantly increasing cyclinB expression and Cdk1 activation. Every bit the cell progresses through G2 and reaches the G2/1000 transition, the kinase Plk1 phosphorylates Wee1, which targets Wee1 for degradation via the SCF ubiquitin ligase complex.[17] An additional part of Plk1 is to activate Cdc25 through phosphorylation. The compound effect of Wee1 degradation and Cdc25 activation is the net removal of inhibitory phosphorylation from cdc2, which activates cdc2. Plk1 is activated at the G2/1000 transition past the Aurora A and Bora, which accumulate during G2 and course an activation complex. The Plk1-Cdc2-cdc25 complex and then initiates a positive feedback loop which serves to further activate Cdc2, and in conjunction with an increase in cyclin B levels during G2, the resulting cdc2-cyclin B complexes so activate downstream targets which promote entry into mitosis.[18] The resultant Cdk1 activeness also activates expression of Mem1-Fkh, a G2/Grand transition cistron.[nineteen] The rapid surge in cyclinB-Cdk1 activity is necessary, every bit G stage initiation is an all-or-nothing event engaging in hysteresis. Hysteresis of Cdk1 activity via cyclin B drives M phase entry past establishing a minimum threshold of cyclinB concentration. This exists at a level college than the minimum needed for the continuation of M phase later on entry, acting to safeguard the all-or-naught event. This entry concentration is further increased in the case of incomplete DNA replication, adding another regulatory machinery at the G2/M transition point.[20] The presence of hysteresis allows for M stage entry to exist highly regulated equally a function of cyclinB-Cdk1 action.
The mechanisms past which mitotic entry is prevented in response to DNA damage are similar to those in the G1/Due south checkpoint. DNA harm triggers the activation of the aforementioned ATM/ATR pathway, in which ATM/ATR phosphorylate and activate the Chk1/Chk2 checkpoint kinases. Chk1/2 phosphorylate cdc25 which, in addition to being inhibited, is also sequestered in the cytoplasm by the fourteen-3-3 proteins. xiv-3-3 are upregulated by p53, which, as previously mentioned, is activated by Chk1 and ATM/ATR. p53 as well transactivates p21, and both p21 and the 14-3-3 in turn inhibit cyclin B-cdc2 complexes through the phosphorylation and cytoplasmic sequestering of cdc2. In addition, the inactivation of cdc25 results in its inability to dephosphorylate and activate cdc2.[21] [22] Finally, another mechanism of damage response is through the negative regulation of Plk1 by ATM/ATR, which in turn results in the stabilization of Wee1 and Myt1, which tin then phosphorylate and inhibit cdc2, thus keeping the jail cell arrested in G2 until the damage is fixed.[23]
G2–Thou transition in Xenopus oocytes [edit]
At the end of G2, the cell transitions into mitosis, where the nucleus divides. The G2 to M transition is dramatic; there is an all-or-aught upshot, and the transition is irreversible. This is advantageous to the cell because entering mitosis is a critical stride in the life cycle of a jail cell. If it does non fully commit, the cell would encounter many bug with partially dividing, ultimately likely leading to the prison cell'south death.
In frog oocytes, the signal pour is induced when progesterone binds to a membrane bound receptor. Downstream, Mos is activated. Mos and then phosphorylates MEK1, which phosphorylates MAPK. MAPK serves two roles: activating the CyclinB-Cdk1 circuitous to initiate entrance into mitosis and activating Mos . The activation of Mos leads to a positive feedback loop and therefore acts every bit "toggle switch" to create the all-or-nix entrance into mitosis.
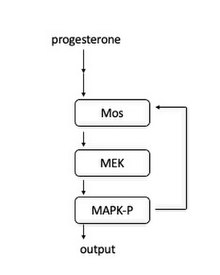
Schematic of the MAPK signaling cascade.
This feedback loop was beginning establish by showing that MAPK-P (phosphorylated MAPK) concentrations increased in response to increasing levels of progesterone.[24] At the single cell level, each cell either had entirely phosphorylated MAPK or no phosphorylated MAPK, confirming that it acts as a switch-like mechanism in each jail cell. It was additionally shown that blocking Mos protein synthesis makes the MAPK-P responses more graded, showing that Mos protein synthesis is necessary for the all-or-none grapheme of MAPK activation.[25]
Bistability [edit]
This process can be understood using bistability. Using the graph shown to the right, the Mos synthesis rate shifts as more than progesterone is added. With each curve, there are stable fixed points and unstable stock-still points. At the unstable fixed points, the system will push button toward either one of the stable fixed points. And then, the system can either exist in the "on" state or the "off" state, non in betwixt. When the progesterone level is high enough, the Mos bend is shifted higher and ultimately intersects the deposition line at only one indicate, and then at that place is merely one stable "on" state, indicating the entrance into mitosis.
The irreversibility we see in the Mitosis transition signal comes from having loftier plenty levels of progesterone in the cell. At high enough levels of progesterone, the arrangement is monostable every bit a result of the positive feedback loop between Mapk and Mos. The point at which the system switches from bistable to monostable is chosen the saddle node bifurcation.
So, nosotros tin can understand the all-or-nothing, irreversible response of the mitotic transition with a mathematical model of the molecular regulators as a bistable organisation that depends on the being of positive feedback. The "off-land" is annihilated past a high enough level of progesterone and once the cell gets pushed past the off-state, information technology is and then stuck in the on-state.
Hysteresis and the Novak–Tyson model [edit]
Coming from this bi-stable model, nosotros can understand the mitotic transition every bit relying on hysteresis to bulldoze it. Hysteresis is defined as the dependence of the country of a system on its history. The Novak–Tyson model is a mathematical model of cell cycle progression that predicts that irreversible transitions entering and exiting mitosis are driven by hysteresis. The model has three bones predictions that should hold true in cycling oocyte extracts whose jail cell cycle progression is dependent on hysteresis:[26]
- The concentration of cyclin B necessary to enter mitosis is higher than the concentration needed to hold a mitotic extract in mitosis.
- Unreplicated DNA raises the level of cyclin necessary for Cdc2 activation and therefore entrance into mitosis.
- At that place is a decrease in the rate of Cdc2 activation at concentrations of cyclin B only above the activation threshold.
Sha et al did experiments in Xenopus laevis egg extracts in 2003 to demonstrate this hysteretic nature.[27] Using cycling extracts, they observed that the activation threshold for Δcyclin B is betwixt 32 and 42 nM whereas the inactivation threshold is between 16 and 24 nM Δcyclin B. Therefore, these experiments confirmed the bistability of this arrangement and the importance of hysteresis in this jail cell cycle transition. At the intermediate cyclin B concentrations, either the interphase or mitotic state of the cell is possible.
Replication stress response [edit]
Since inbound mitosis is a large and plush commitment for the jail cell, it is logical that systems would be in identify to prevent premature entrance into this step. Information technology has been shown that mistakes in previous steps, such equally having unreplicated sections of DNA blocks progression in the cell cycle.[28] The Novak–Tyson model predicts this occurs via raising the level of cyclin B necessary for archway into mitosis.[26]
Sha et al. investigated whether this was true in Xenopus egg extracts. They used aphidicolin (APH) to inhibit DNA polymerase and prevent Dna replication. When treated with Cyclin B in interphase, the threshold of activation increased to between 80 and 100 nM, as predicted past the Novak–Tyson model.[27] So, these experiments confirm that the stress of unreplicated DNA in the prison cell bear upon the hysteresis loop and event in a much higher cyclin B threshold to enter into mitosis.
Metaphase checkpoint [edit]
The mitotic spindle checkpoint occurs at the signal in metaphase where all the chromosomes should/take aligned at the mitotic plate and be under bipolar tension. The tension created by this bipolar attachment is what is sensed, which initiates the anaphase entry. To practise this, the sensing machinery ensures that the anaphase-promoting complex (APC/C) is no longer inhibited, which is now free to dethrone cyclin B, which harbors a D-box (devastation box), and to pause down securin.[29] The latter is a protein whose function is to inhibit separase, which in turn cuts the cohesins, the poly peptide composite responsible for cohesion of sister chromatids.[thirty] In one case this inhibitory protein is degraded via ubiquitination and subsequent proteolysis, separase then causes sister chromatid separation.[31] After the cell has split into its two daughter cells, the prison cell enters G1.
Cancer [edit]
Dna repair processes and cell cycle checkpoints have been intimately linked with cancer due to their functions regulating genome stability and cell progression, respectively. The precise molecular mechanisms that connect dysfunctions in these pathways to the onset of particular cancers are not well understood in most cases.[32] The loss of ATM has been shown to precede lymphoma development presumably due to excessive homologous recombination, leading to high genomic instability.[33] Disruption of Chk1 in mice led pregnant misregulation of cell cycle checkpoints, an accumulation of Deoxyribonucleic acid damage, and an increased incidence of tumorigenesis.[34] Perhaps virtually famously, single mutant inheritance of BRCA1 or BRCA2 predisposes females toward breast and ovarian cancers.[35] BRCA1 is known to be required for Southward and G2/M transitions, and is involved in the cellular response to DNA damage. BRCA2 is believed to be involved in homologous recombination and regulating the Southward-stage checkpoint, and mutations of deficiencies in BRCA2 are strongly linked to tumorigenesis.[36]
See also [edit]
- Biochemical switches in the prison cell cycle
- Prison cell cycle analysis
- G2-M DNA harm checkpoint
- Postreplication checkpoint
- Meiotic recombination checkpoint
References [edit]
- ^ Hartwell, Fifty.; Weinert, T. (3 November 1989). "Checkpoints: controls that ensure the society of cell cycle events". Science. 246 (4930): 629–634. Bibcode:1989Sci...246..629H. doi:10.1126/science.2683079. ISSN 0036-8075. PMID 2683079.
- ^ Morgan, David Owen (1958–2007). The jail cell bicycle : principles of control. London: New Science Press. ISBN978-0-19-920610-0. OCLC 70173205.
- ^ Murray, A.; Kirschner, M. (3 Nov 1989). "Dominoes and clocks: the union of two views of the cell cycle". Science. 246 (4930): 614–621. Bibcode:1989Sci...246..614M. doi:ten.1126/scientific discipline.2683077. ISSN 0036-8075. PMID 2683077.
- ^ Morgan, David O. (November 1997). "CYCLIN-DEPENDENT KINASES: Engines, Clocks, and Microprocessors". Annual Review of Cell and Developmental Biology. 13 (ane): 261–291. doi:10.1146/annurev.cellbio.13.1.261. ISSN 1081-0706. PMID 9442875.
- ^ a b c Alberts B, Johnson A, Lewis J, Raff M, Roberts K (2007). Molecular biology of the cell (fifth ed.). New York: Garland Science. ISBN9780815341055.
- ^ Cooper GM (2000). The cell : a molecular approach (2nd ed.). Washington (DC): ASM Press. ISBN978-0-87893-106-iv.
- ^ Lodish H, Baltimore D, Berk A (2000). Molecular jail cell biology (fourth ed.). New York: Scientific American Books. ISBN978-0-7167-3136-8.
- ^ Malumbres Chiliad, Barbacid Chiliad (March 2009). "Prison cell cycle, CDKs and cancer: a changing image". Nature Reviews. Cancer. 9 (3): 153–66. doi:10.1038/nrc2602. PMID 19238148. S2CID 2613411.
- ^ Vermeulen M, Van Bockstaele DR, Berneman ZN (June 2003). "The cell cycle: a review of regulation, deregulation and therapeutic targets in cancer". Prison cell Proliferation. 36 (iii): 131–49. doi:10.1046/j.1365-2184.2003.00266.x. PMC6496723. PMID 12814430.
- ^ a b c d east Bertoli C, Skotheim JM, de Bruin RA (August 2013). "Control of cell cycle transcription during G1 and Southward phases". Nature Reviews Molecular Cell Biology. 14 (8): 518–28. doi:10.1038/nrm3629. PMC4569015. PMID 23877564.
- ^ a b c Narasimha AM, Kaulich M, Shapiro GS, Choi YJ, Sicinski P, Dowdy SF (June 2014). "Cyclin D activates the Rb tumor suppressor by mono-phosphorylation". eLife. three. doi:10.7554/eLife.02872. PMC4076869. PMID 24876129.
- ^ Skotheim JM, Di Talia Due south, Siggia ED, Cross FR (July 2008). "Positive feedback of G1 cyclins ensures coherent prison cell cycle entry". Nature. 454 (7202): 291–6. Bibcode:2008Natur.454..291S. doi:10.1038/nature07118. PMC2606905. PMID 18633409.
- ^ Bartek J, Lukas J (Dec 2001). "Mammalian G1- and S-phase checkpoints in response to DNA damage". Current Opinion in Cell Biology. thirteen (vi): 738–47. doi:ten.1016/S0955-0674(00)00280-5. PMID 11698191.
- ^ Bertoli C, de Bruin RA (July 2014). "Turning cell cycle entry on its head". eLife. iii: e03475. doi:x.7554/eLife.03475. PMC4076868. PMID 24986860.
- ^ Morgan D (2007). The Cell Cycle Principles of Control. New Scientific discipline Press. pp. 92–95.
- ^ Morgan D (2007). The Jail cell Cycle Principles of Command. New Science Printing. pp. 228–229.
- ^ Guardavaccaro D, Pagano Chiliad (Apr 2006). "Stabilizers and destabilizers decision-making cell cycle oscillators". Molecular Cell. 22 (1): ane–4. doi:x.1016/j.molcel.2006.03.017. PMID 16600864.
- ^ Seki A, Coppinger JA, Jang CY, Yates JR, Fang One thousand (June 2008). "Bora and the kinase Aurora a cooperatively activate the kinase Plk1 and command mitotic entry". Scientific discipline. 320 (5883): 1655–eight. Bibcode:2008Sci...320.1655S. doi:ten.1126/science.1157425. PMC2834883. PMID 18566290.
- ^ Morgan D (2007). The Jail cell Cycle Principles of Control. New Science Press. pp. 44–45, xc.
- ^ Sha W, Moore J, Chen G, Lassaletta AD, Yi CS, Tyson JJ, Sible JC (February 2003). "Hysteresis drives cell-cycle transitions in Xenopus laevis egg extracts". Proceedings of the National University of Sciences of the United States of America. 100 (3): 975–80. Bibcode:2003PNAS..100..975S. doi:x.1073/pnas.0235349100. PMC298711. PMID 12509509.
- ^ Wang Y, Ji P, Liu J, Broaddus RR, Xue F, Zhang W (February 2009). "Centrosome-associated regulators of the Thousand(2)/M checkpoint as targets for cancer therapy". Molecular Cancer. 8 (1): viii. doi:x.1186/1476-4598-viii-8. PMC2657106. PMID 19216791.
- ^ Löbrich M, Jeggo PA (November 2007). "The bear upon of a negligent G2/Thousand checkpoint on genomic instability and cancer induction". Nature Reviews. Cancer. 7 (11): 861–ix. doi:10.1038/nrc2248. PMID 17943134. S2CID 30207932.
- ^ Harper JW, Elledge SJ (December 2007). "The DNA damage response: ten years after". Molecular Prison cell. 28 (v): 739–45. doi:10.1016/j.molcel.2007.xi.015. PMID 18082599.
- ^ Gotoh, Yukiko; Masuyama, Norihisa; Dell, Karen; Shirakabe, Kyoko; Nishida, Eisuke (October 1995). "Initiation of Xenopus Oocyte Maturation by Activation of the Mitogen-activated Poly peptide Kinase Cascade". Journal of Biological Chemical science. 270 (43): 25898–25904. doi:10.1074/jbc.270.43.25898. ISSN 0021-9258. PMID 7592777.
- ^ Ferrell Jr., J. East. (1998-05-08). "The Biochemical Basis of an All-or-None Jail cell Fate Switch in Xenopus Oocytes". Science. 280 (5365): 895–898. Bibcode:1998Sci...280..895F. doi:10.1126/science.280.5365.895. ISSN 0036-8075. PMID 9572732.
- ^ a b Novak, B.; Tyson, J.J. (1993-12-01). "Numerical analysis of a comprehensive model of M-stage command in Xenopus oocyte extracts and intact embryos". Journal of Cell Science. 106 (iv): 1153–1168. doi:10.1242/jcs.106.four.1153. ISSN 1477-9137. PMID 8126097.
- ^ a b Sha, Westward.; Moore, J.; Chen, Chiliad.; Lassaletta, A. D.; Yi, C.-S.; Tyson, J. J.; Sible, J. C. (2002-12-30). "Hysteresis drives prison cell-cycle transitions in Xenopus laevis egg extracts". Proceedings of the National University of Sciences. 100 (3): 975–980. doi:10.1073/pnas.0235349100. ISSN 0027-8424. PMC298711. PMID 12509509.
- ^ Dasso, Mary; Newport, John Westward. (June 1990). "Completion of DNA replication is monitored by a feedback system that controls the initiation of mitosis in vitro: Studies in Xenopus". Cell. 61 (5): 811–823. doi:10.1016/0092-8674(90)90191-thou. ISSN 0092-8674. PMID 2160859. S2CID 34852886.
- ^ Peters JM (December 1998). "SCF and APC: the Yin and Yang of jail cell wheel regulated proteolysis". Electric current Opinion in Jail cell Biological science. 10 (6): 759–68. doi:10.1016/S0955-0674(98)80119-1. PMID 9914180.
- ^ Ciosk R, Zachariae Due west, Michaelis C, Shevchenko A, Mann M, Nasmyth K (June 1998). "An ESP1/PDS1 complex regulates loss of sister chromatid cohesion at the metaphase to anaphase transition in yeast". Cell. 93 (6): 1067–76. doi:x.1016/S0092-8674(00)81211-eight. PMID 9635435. S2CID 9951929.
- ^ Karp G (2005). Prison cell and Molecular Biology: Concepts and Experiments (4th ed.). Hoboken, New Jersey: John Wiley and Sons. pp. 598–9. ISBN978-0-471-16231-five.
- ^ Kastan MB, Bartek J (November 2004). "Cell-bicycle checkpoints and cancer". Nature. 432 (7015): 316–23. Bibcode:2004Natur.432..316K. doi:x.1038/nature03097. PMID 15549093. S2CID 4415666.
- ^ Shiloh Y, Kastan MB (2001). "ATM: genome stability, neuronal evolution, and cancer cross paths". Advances in Cancer Research. 83: 209–54. doi:10.1016/s0065-230x(01)83007-iv. ISBN9780120066834. PMID 11665719.
- ^ Lam MH, Liu Q, Elledge SJ, Rosen JM (July 2004). "Chk1 is haploinsufficient for multiple functions critical to tumor suppression". Cancer Cell. 6 (1): 45–59. doi:10.1016/j.ccr.2004.06.015. PMID 15261141.
- ^ King MC, Marks JH, Mandell JB (October 2003). "Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2". Science. 302 (5645): 643–6. Bibcode:2003Sci...302..643K. doi:ten.1126/science.1088759. PMID 14576434. S2CID 33441900.
- ^ Venkitaraman AR (Jan 2002). "Cancer susceptibility and the functions of BRCA1 and BRCA2". Cell. 108 (two): 171–82. doi:10.1016/s0092-8674(02)00615-3. PMID 11832208. S2CID 10397442.
When Does The Cytoplasm Divide,
Source: https://en.wikipedia.org/wiki/Cell_cycle_checkpoint
Posted by: edwardsmajected1995.blogspot.com


0 Response to "When Does The Cytoplasm Divide"
Post a Comment